Mexidol® and Mexidol® FORTE 250 are reference (original) preparations
LLC “Scientific Production Company “PHARMASOFT” reports receipt of a letter (No. 20-3/545 of 13.04.2020) from the Department for State Regulation of the Circulation of Medicines of the Ministry of Health of the Russian Federation which confirms that the following drugs are reference (original) ones1: Mexidol® in the pharmaceutical form “film-coated tablets, 125 mg” (LSR-002063/07 of 09.08.2007) and "solution for intravenous and intramuscular administration, 50 mg/mm (P N002161/0l of 14.03.2008), as well as Mexidol® FORTE 250 in the pharmaceutical form “film-coated tablets, 250 mg” (LP-004831 of 26.04.2018) (owner or holder of the registration certificate - LLC “Scientific Production Company “PHARMASOFT”, Russia).
1 Letter from the Federal State Budgetary Institution “Scientific Centre for Expert Evaluation of Medicinal Products” of the Ministry of Health of Russia No. 7358 dated 07.04.2020.
When a great victory is needed
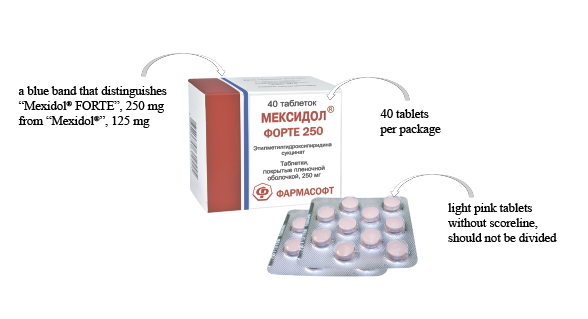
LLC "SPC "PHARMASOFT" announces registration of a new dosage of the drug Mexidol® - Mexidol® FORTE 250, film-coated tablets, 250 mg, RU No. LP-004831 of April 26, 2018.
"Mexidol® FORTE 250" is a double dosage of the active ingredient in one film-coated tablet for patients who require an increased dosage, including patients with combined pathology. "Mexidol® FORTE 250" is a reference (original) preparation of ethylmethylhydroxypyridine succinate, the only preparation with active substance ethylmethylhydroxypyridine succinate with a double dosage. "Mexidol® FORTE 250" - a high safety profile.
Presentation of the preparation "Mexidol® FORTE 250" to broad medical community was within the framework of the Xth International Congress "Neurorehabilitation 2018", which was held on May 31 - June 01, 2018 in the building of the Mayor's office of the Moscow Government at the address: Novy Arbat str., 36, at the Simposium "Peculiarities of Management & Rehabilitation of Patients with Comorbid Vascular Diseases", June 01, 9:00-10:30, Sector C.
Results of the most important study on effectiveness and safety of Mexidol (EPICA) have been published
LLC "SPC "PHARMASOFT" informed about completion of randomized, double-blind, multicenter, placebo-controlled in parallel groups study of effectiveness and safety of Mexidol in long-term sequential therapy in patients with hemispheric ischemic stroke EPICA in acute and early rehabilitation periods. The results of the study have been published (Stakhovskaya L.V., Shamalov N.A., Khasanova D.R., Melnikova E.V., et al. Journal of Neurology & Psychiatry named after S.S. Korsakov2017; 3 (2):55-65; Supplement "Stroke").
Objective of the Research. To evaluate effectiveness and safety of long-term sequential therapy with preparation of Mexidol in patients with hemispheric ischemic stroke (IS) in acute and early rehabilitation periods.
Material and Methods. In a randomized, double-blind, multicenter, placebo-controlled study in parallel groups, 151 patients (62 men and 89 women) were enrolled, 150 patients (62 men and 88 women) aged 40 to 79 years were randomized. The patients were randomly assigned to 2 groups: patients of the 1st group received mexidol 500 mg/day by intravenously drop infusion for 10 days, followed by 1 tablet (125 mg) 3 times per day for 8 weeks. The patients of the 2nd group received a placebo according to similar scheme. The duration of participation in the study was 67 to 71 days.
Results. At the end of the therapy, the mean score by the modified Rankine scale (mSHR) was lower in the 1st group than in the 2nd one (p = 0.04). Dynamics of decrease in average score by mRS (1-5th visits) was more evident in the 1st group (p = 0.023). The proportion of patients, who achieved a recovery, corresponding to 0-2 points by mRS (5th visit) was significantly higher in the 1st group (p=0.039). When testing by Stroke Scale of National Institute of Health at the 5th visit, the mean value was lower in the 1st group (p=0.035). The decrease in score by Stroke Scale of National Institute of Health at the end of the course of the therapy relative to baseline in patients with diabetes mellitus was more pronounced in the 1st group (p=0.038).
In the 1st group, in general population of patients and subpopulation of patients with diabetes mellitus, the dynamics of improving quality of life was more evident and was observed since the 2nd visit.
Separate analysis of functions by EQ-5D questionnaire (health scale "movement") in both treatment groups revealed a significant (p <0.001) linear dependence towards an increase in the number of patients with no problems with movement, as well as statistically significant difference between groups of the second and fifth visits. It was found that 53 (86.9%) patients of the 1st group noted that they had no problems with movement; 48 (78.7%) - noted that they had no problems with self-care; 43 (70.5%) - considered that they had no problems with everyday activities (work, study, household chores, family obligations, leisure activities); 52 (85.2%) - did not feel pain and discomfort; 54 (88.5%) - did not experience anxiety and depression.
In assessing safety, the tolerability of therapy with Mexidol and placebo is regarded as satisfactory. There were no significant differences in rate of adverse events in patients of both groups.
The obtained data indicate a comparable safety profile for preparation Mexidol® and placebo, when used in patients in acute and early recovery periods of hemispheric IS.
Conclusion. It is recommended to include preparation Mexidol® into the treatment of patients in acute and early recovery periods of ischemic stroke.
1 Journal of Neurology & Psychiatry. 2017; 117: 3 (2): 55-65.
The Journal is presented in the following international databases: Russian Science Citation Index, Web of Science (Russian Science Citation Index — RSCI), PubMed/Medline, Index Medicus, Ulrich’s Periodicals Directory, Scopus/EMBASE, Google Scholar.